
NovaGRAFT Cancellous Bone Graft
For regeneration of bone defects
Button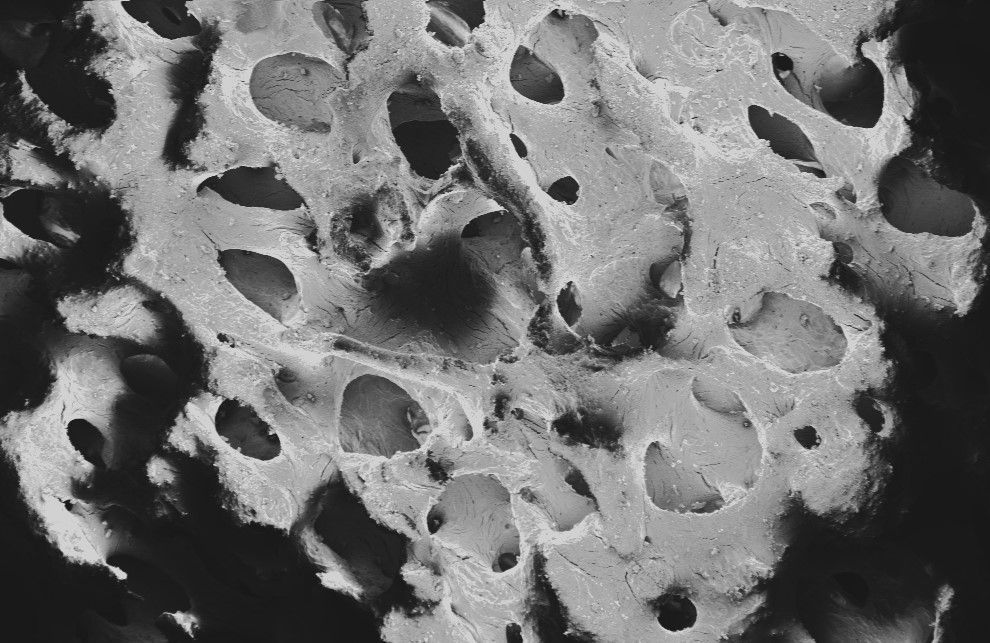
Trabecular structure
For optimal perfusion and host cell attachment and proliferation
Button
NovaGRAFT
Available in all sizes for your surgical needs
Button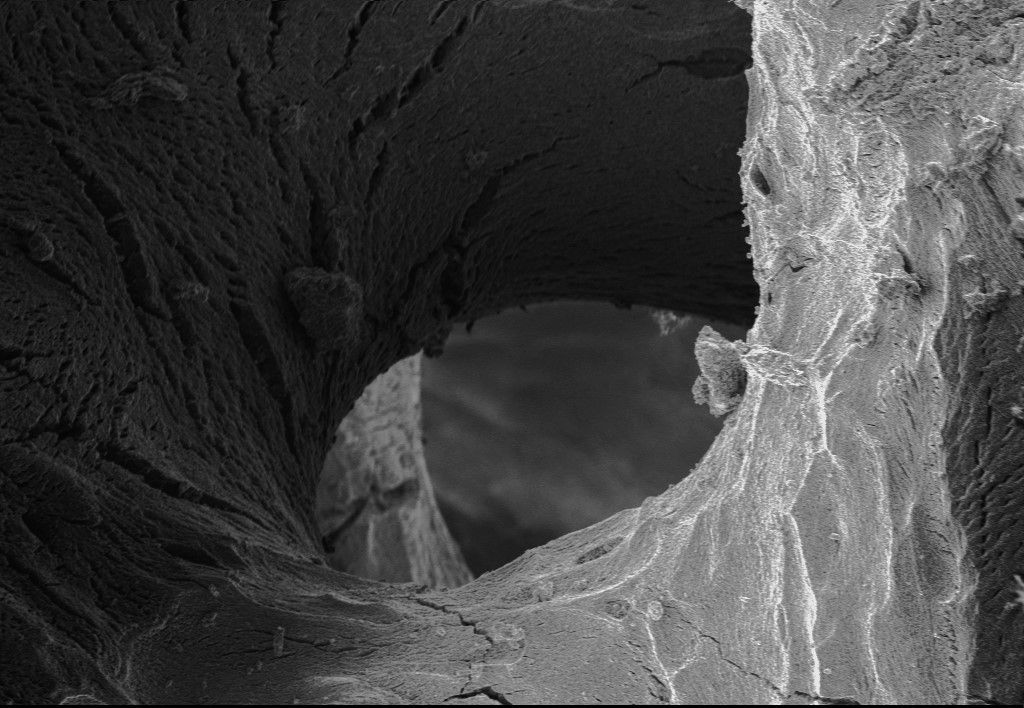
Optimal Porosity
For complete integration with host tissue
Button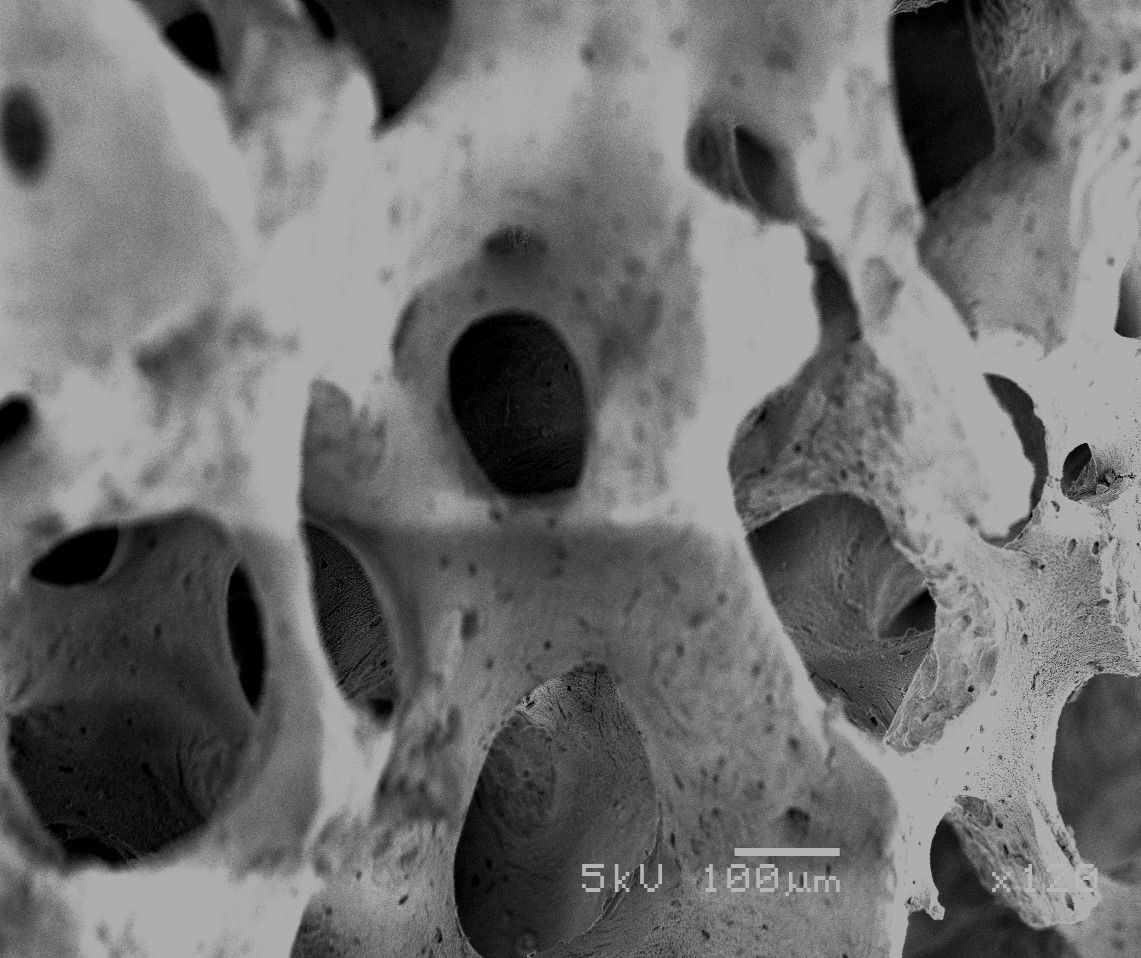
Native Bone Structure
Resobrs over 6 months leaving healthy de novo host bone
Button
NovaGRAFT is was developed as a result of our collaboration with specialist surgeons who identified the need for a standardized-BMP content graft material for periodontal, maxillo-facial and reconstructive uses. NovaGRAFT consists of demineralized bone matrix of porcine origin that has been made biocompatible through our proprietary enzymatic, chemical and physical treatments. There are no cellular remnants, nucleic acid residues or antigenic epitopes present after processing. The graft is dehydrated and terminally gamma-irradiated before undergoing final QC testing. Every lot undergoes twelve different physical, molecular, microbiological and antibody tests which ensures every lot is identical. This rigorous testing ensures a reliable and predictable clinical outcome.
The material completely resorbs over six months after it has fulfilled its role as being a template for de novo host bone which has been histologically proven to induce bone formation by matrix deposition. Hence it is not a prosthetic, not a cement. The only thing that remains after healing is you.


