Novatend’s cutting-edge tissue engineering platform provides a full suite of bone grafting and biomaterial solutions designed to meet your surgical needs.
Learn About Us...
NEXT-GENENRATION
TISSUE-ENGINEERING
Novatend uses its knowledge of tissue engineering and medical device manufacturing to provide the most affordable high-quality biomaterial grafting solutions in the world.
LEARN MORE

MOST POPULAR DEVICES
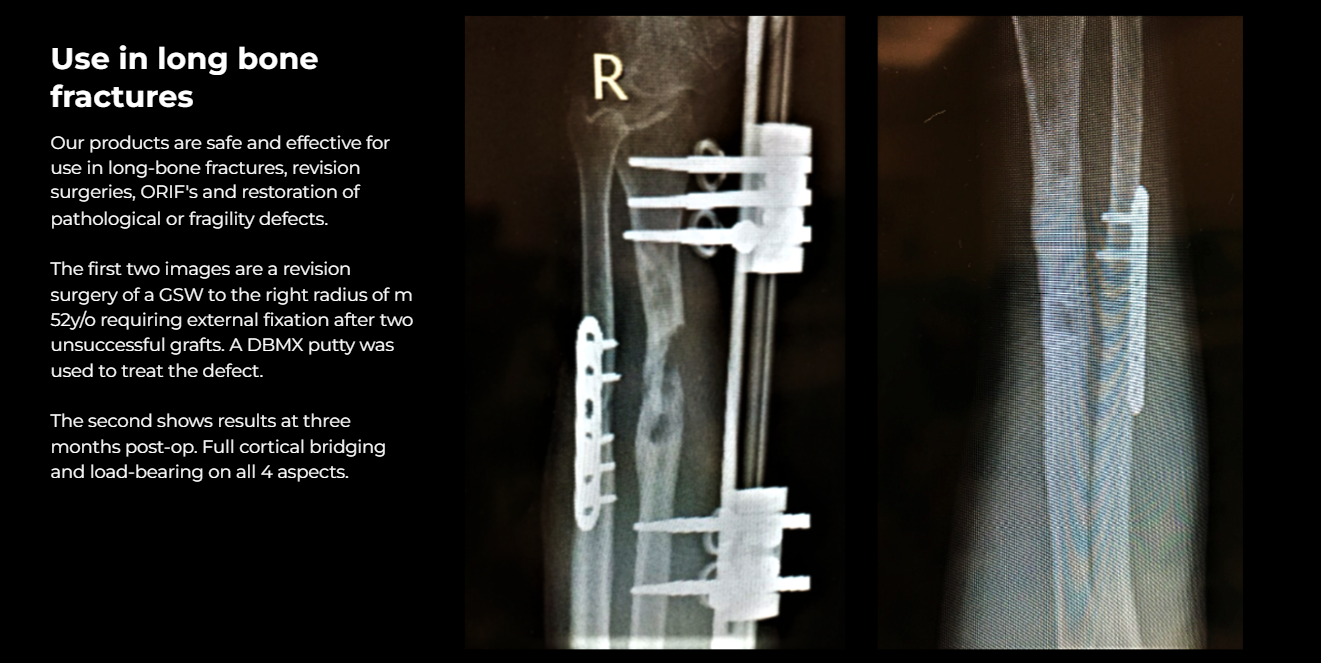
All devices are high-quality locally manufactured grafts that yield predictable and reliable clinical outcomes.

NovaDBMX
Ready-to-inject osteoinductive putty containing high levels of BMP-complex for spinal fusions, ORIFS and non-unions

NovaGROW
DBM Granules with standardized BMP levels for sinus lifts, ridge augmentations and socket preservations

NovaMEM
Atelocollagen barrier membrane for graft sequestration/tissue approximation

NovaGRAFT
Biocompatible spongiosa for reconstructive procedures

NovatendX
Bioresorbable rotator cuff patch graft for the augmentation of traditional repairs

NovaSGX
Fully synthetic ready-to-inject graft for maxillofacial and periodontal procedures
NEXT-GEN INJECTABLE
BONE GRAFTING
We provide the latest in bone grafting materials straight to our customers. This enables shorter time spent in theater, reduced risk due to disease-transfer and infection and for a fraction of the price of a second procedure
Get Started

We have built a great platform in which we can provide cutting edge regenerative medicine based solutions directly to distributors, hospital groups and surgeons. Our devices sell at a fraction of the cost of imported competitors and are fully reimbursed by major medical aid groups, giving patients a much needed break from inflated out-of-pocket expenses.
- MC Labuschagne, Executive Director
2241
Succesful Operations
16.4%
Month on Month Growth
Q1-Q4 2025
0
Adverse Events Reported